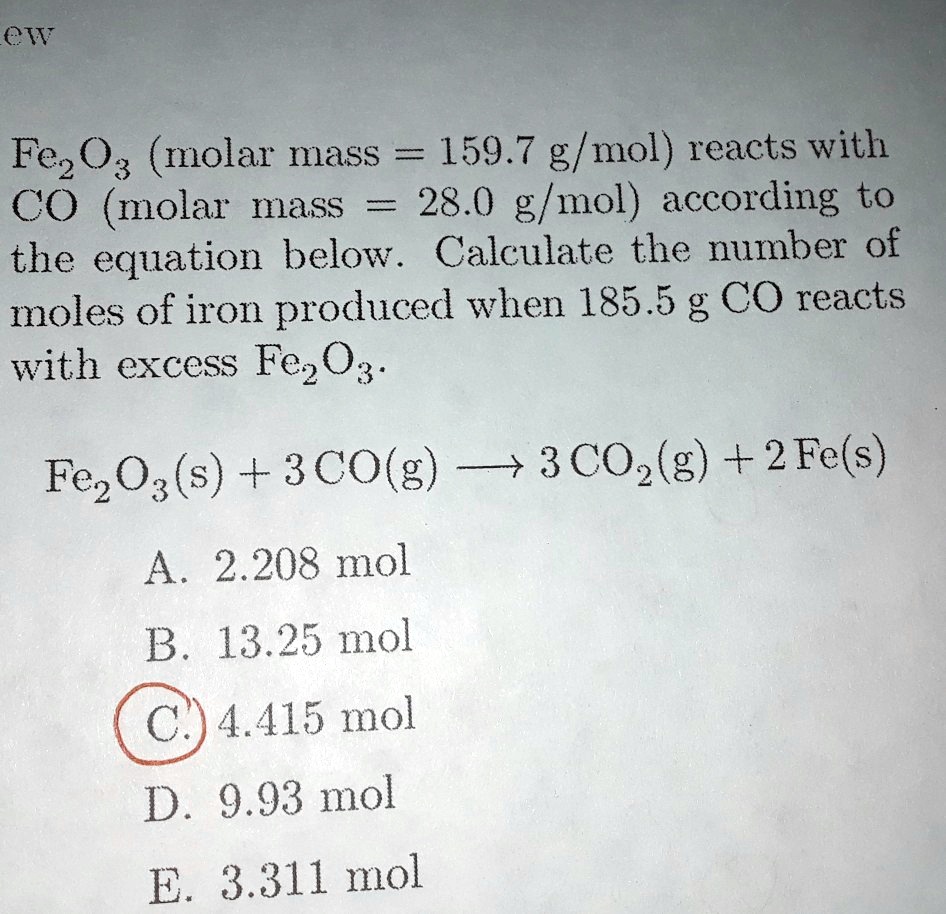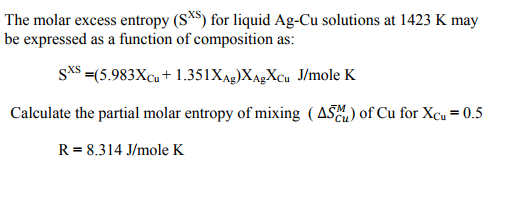SciELO - Brasil - DENSITY, REFRACTIVE INDEX, APPARENT VOLUMES AND EXCESS MOLAR VOLUMES OF FOUR PROTIC IONIC LIQUIDS + WATER AT T=298.15 AND 323.15 K DENSITY, REFRACTIVE INDEX, APPARENT VOLUMES AND EXCESS
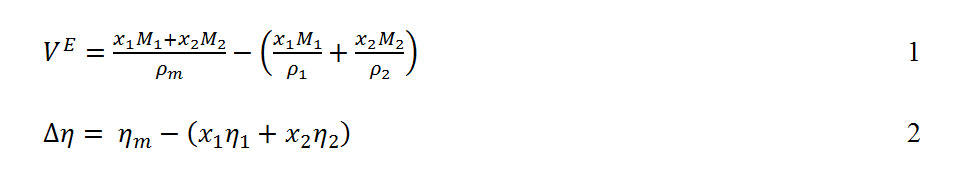
Derived thermodynamic properties of binary mixtures of m-Xylene, o-Xylene, and p-Xylene, with N,N-Dimethylformamide at T = (293.15, 303.15, 313.15 and 323.15) K : Oriental Journal of Chemistry
Limiting Reactants & Excess. Limiting Reactant Calculations In many chemical reactions an excess of one reactant is added to ensure complete reaction. - ppt download

200 mol of ethane are burned in a furnace with 50% excess air. A conversion of 95% is achieved. Calculate the composition of the stack gases?

OneClass: Acetylene gas (C2H2) is burned completely with 20 percent excess air during a steady state-...

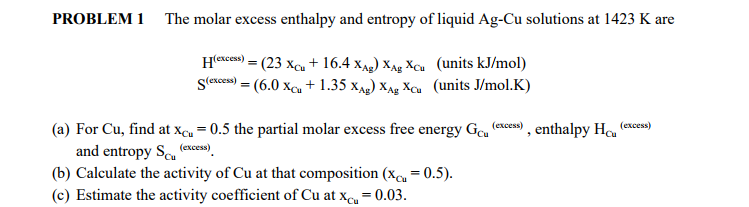
SOLVED: The molar excess Gibbs free energy of formation of solid solutions in the 'system Au-Ni can be represented by G*s '=XiXAu(QAIAOX Au + 38280X Ni 14230XAuX Ni } 2660 _ Calculate

molar gas volume Avogadro's Law moles and mass calculations gcse chemistry calculations igcse KS4 science A level GCE AS A2 O Level practice questions exercises