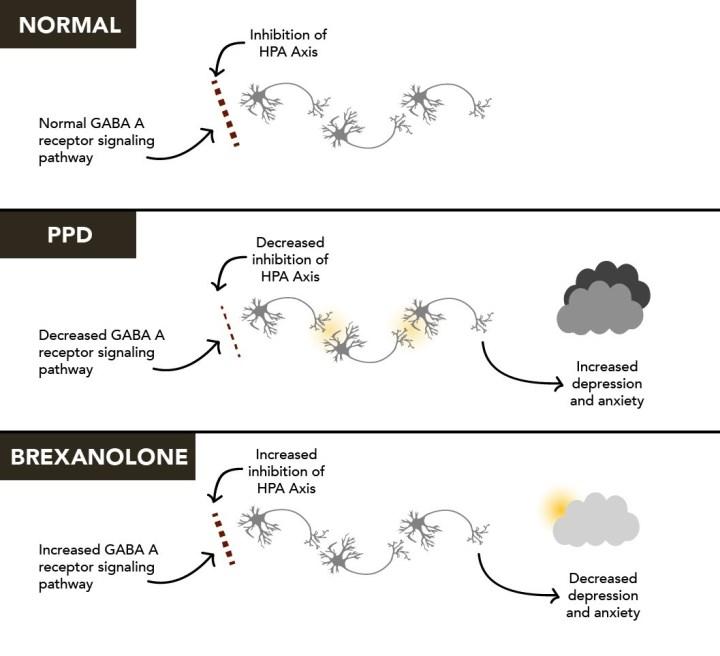
Sage Therapeutics Receives FDA Breakthrough Therapy Designation for SAGE-217 for the Treatment of Major Depressive Disorder - Chemdiv
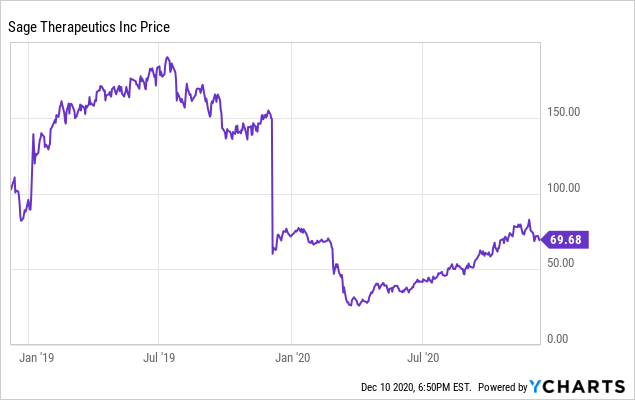
Sage Therapeutics: Zuranolone's Multi-Billion Dollar Market Opportunity More Than Justifies Company Valuation (NASDAQ:SAGE) | Seeking Alpha
Discovering, developing and delivering life-changing therapies to treat rare central nervous system disorders.
Sage Therapeutics Forges $575 Million Deal With Shionogi to Market Depression Drug in Parts of Asia | BioSpace

SY Investing on Twitter: "$SAGE (-62% PM) announced pivotal Ph 3 MOUNTAIN results of SAGE-217 in MDD. Study didn't meet primary endpoint. #Fail SAGE- 217 showed mean reduction of 12.6 in HAM-D total

Biogen and Sage Therapeutics Complete Rolling Submission of New Drug Application for Zuranolone in the Treatment of Major Depressive Disorder and Postpartum Depression | Biogen

Sage Therapeutics Announces Development Plan for Zuranolone (SAGE-217) Following Breakthrough Therapy Guidance Meeting with the U.S. Food & Drug Administration | Business Wire

Sage Therapeutics and Biogen Initiate Rolling Submission of New Drug Application (NDA) to U.S. Food and Drug Administration for Zuranolone for the Potential Treatment of Major Depressive Disorder (MDD) | Business Wire